With rapid economic development and rising living standards in our country, the national fondness for sweets and sugary beverages has been on the rise [1]. Since the 1970s, high-fructose corn syrup (HFCS), due to its high sweetness, easy availability, and low cost, has gradually replaced sucrose to become one of the primary sweeteners in the food processing industry for products such as candies, cakes, and soft drinks [2]. Increasing evidence suggests that long-term consumption of high amounts of fructose can lead to visceral fat accumulation, dysregulation of Sugar and lipid metabolism, oxidative stress, and chronic mild inflammation, becoming a risk factor for metabolic syndromes such as obesity, cardiovascular disease, non-alcoholic fatty liver disease, and insulin resistance [3-4]. Therefore, finding safe and effective dietary approaches to prevent metabolic syndromes induced by high-fructose diets has become an urgent issue in the fields of food nutrition science and health.
As a functional food and beverage with health-promoting properties, Tea is widely consumed. Epigallocatechin gallate (EGCG), one of the most abundant polyphenolic components in Green Tea, has been shown to effectively alleviate metabolic disorders [5-6] and have anti-inflammatory effects [7-8]. This article, based on animal experiments, finds that EGCG can prevent metabolic disorders caused by a high-fructose diet through reducing lipid accumulation, stabilizing blood sugar regulation, repairing the intestinal barrier, and inhibiting inflammatory responses.
I. Improvement Effects of EGCG on Body Weight, Tissue Characteristics, and Glucose Regulation in Mice Fed a High-Fructose Diet
EGCG can effectively prevent excessive lipid accumulation and stabilize blood sugar regulation. Researchers found that dietary supplementation with 1% EGCG, without affecting the food intake or daily activities of mice, reduces excessive lipid accumulation and fat mass, alleviating weight gain in mice fed a high-fructose diet. Additionally, it slows down the increase in blood sugar levels and shortens the time required for blood sugar levels to return to normal in these mice.
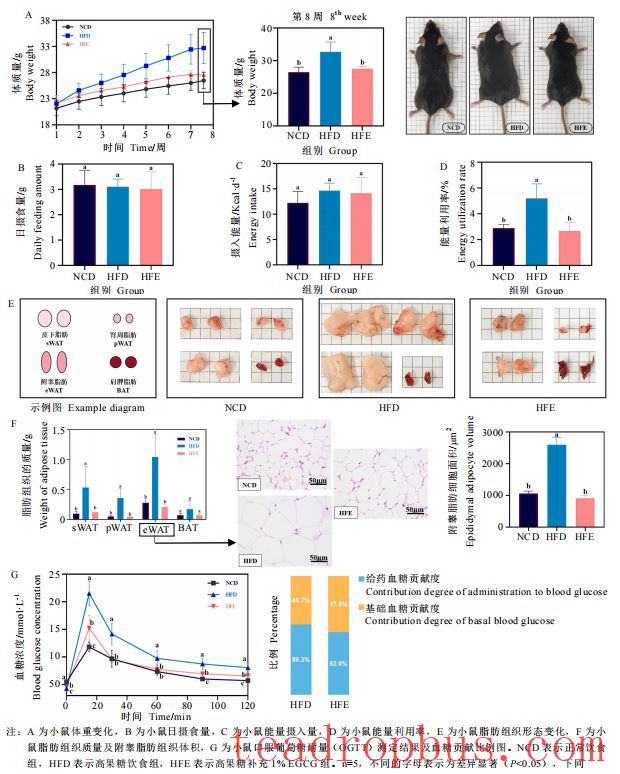
II. Improvement Effects of EGCG on Liver and Intestinal Characteristics and Inflammatory Responses in Mice Fed a High-Fructose Diet
EGCG can effectively maintain liver and intestinal functions under a high-fructose diet background. The liver and intestines are major organs that play crucial roles in stabilizing blood sugar and lipid levels and regulating lipid metabolism. Researchers found that dietary supplementation with 1% EGCG significantly reduced the liver weight in mice fed a high-fructose diet, similar to the normal group; it also significantly decreased the size of lipid droplets and vacuoles in the liver, improving hepatic steatosis. Similarly, researchers observed that the intestinal walls in mice fed a high-fructose diet were significantly thinner, with submucosal edema, disappearance of glandular cavities, mostly incomplete glands, and disordered arrangement, indicating significant structural damage; however, mice fed EGCG had more intact intestinal gland structures, improved glandular cavity arrangement, and only a few lost goblet cells. Furthermore, EGCG reduced the levels of TNF-α and IL-1β inflammatory factors in the liver and IL-6 inflammatory factors in the intestines.
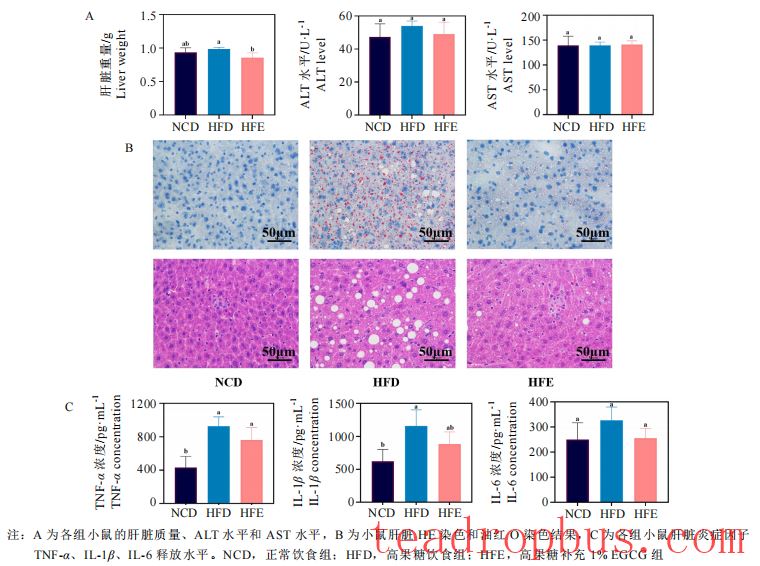
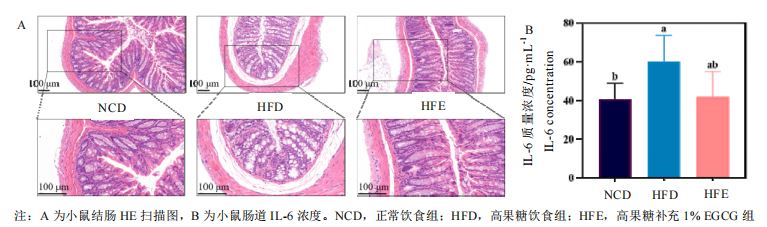
III. Impact of EGCG on Key Gene Expression Related to Lipid Metabolism and Inflammatory Response Pathways in Mice Fed a High-Fructose Diet
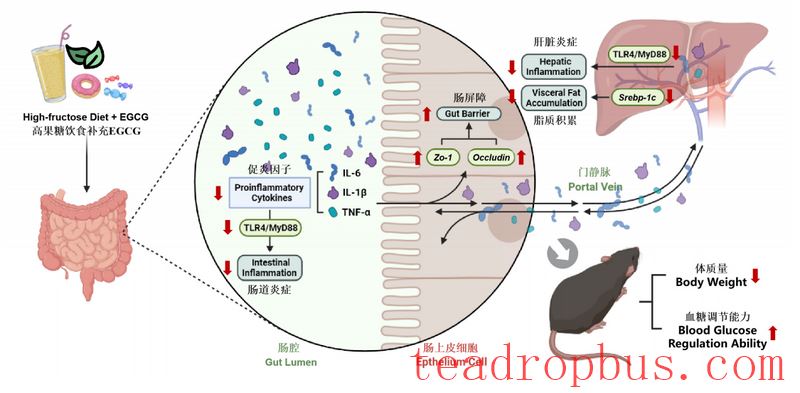
The liver and intestines play critical roles in fructose metabolism, connecting nutrients and metabolic products through the portal venous system. We typically refer to this bidirectional communication pathway as the “liver-intestine axis” [9].
The intestinal barrier is the foundation for the functioning of the intestines. ZO-1 and Occludin are key proteins that promote tight junctions in intestinal epithelial cells and are closely related to intestinal permeability. The TLR4/MyD88 signaling pathway is an important pathway mediating inflammatory responses. After TLR4 recognizes corresponding signals, it transmits intracellular signals through downstream signaling molecules MyD88, leading to the activation of nuclear factor-κB (NF-κB), which promotes the secretion of pro-inflammatory cytokines such as TNF-α, IL-6, IL-8, and IL-1β, inducing inflammation [10-11]. Researchers found that EGCG may regulate lipid metabolism disorders caused by a high-fructose diet by suppressing the expression of the liver lipid metabolism gene Srebp-1c, increasing the expression level of the Zo-1 gene, and the expression levels of ZO-1 and Occludin proteins to ensure the structure and function of the intestine, and together downregulating the expression levels of the Tlr4 and Myd88 genes in the liver and intestines to improve inflammation.
In summary, dietary supplementation with EGCG can effectively improve weight gain, lipid accumulation, blood sugar disorders, and liver dysfunction in mice fed a high-fructose diet. It can upregulate the expression levels of the Zo-1 gene, ZO-1, and Occludin proteins to strengthen intestinal barrier function and downregulate the levels of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α in the liver and intestines to improve inflammatory responses. Its mechanism of action may be related to the inhibition of the TLR4/MyD88 signaling pathway. Researchers have revealed the role and mechanism of dietary supplementation with EGCG in improving metabolic disorders induced by a high-fructose diet from the perspective of the synergistic regulation of dietary polyphenol EGCG and the gut-liver axis, providing experimental evidence and research foundations for the prevention and treatment of high-fructose-related metabolic syndromes and the development of related functional foods.
References
[1]Ren Z B, Xu P P, Zhang Q, et al. Relationship between sweet food intake and myopia in Chinese children aged 11-14 years from 2025 to 2025 [J]. Chinese Journal of Public Health, 2025, 51(5): 713-719.
[2]Hattori H, Hanai Y, Oshima Y, et al. Excessive intake of high-fructose corn syrup drinks induces impaired glucose tolerance [J]. Biomedicines, 2025, 9(5): 541. doi:10.3390/biomedicines9050541.
[3]Janevski M, Ratnayake S, Siljanovski S, et al. Fructose-containing sugars modulate mRNA of lipogenic genes ACC and FAS and protein levels of transcription factors ChREBP and SREBP1c with no effect on body weight or liver fat [J]. Food & Function, 2012, 3(2): 141-149.
[4]Softic S, Meyer J G, Wang G X, et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins [J]. Cell Metabolism, 2025, 30(4): 735-753.
[5]Ren Z K, Yang Z Y, Lu Y, et al. Anti-glycolipid disorder effect of epigallocatechin3gallate on high-fat diet and STZ-induced T2DM in mice [J]. Molecular Medicine Reports, 2025, 21(6): 2475-2483.
[6]Xu L L, Li W W, Chen Z Q, et al. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypogly